A compound ‘X’ on heating with excess conc. sulphuric acid at 443 K gives an unsaturated compound
Carbon Compounds (10)A compound ‘X’ on heating with excess conc. sulphuric acid at 443 K gives an unsaturated compound ‘Y’. ‘X’ also reacts with sodium metal to evolve a colourless gas ‘Z’. Identify ‘X’, ‘Y’ and ‘Z’. Write the equation of the chemical reaction of formation of ‘Y’ and also write the role of sulphuric acid in the reaction.
Answer
- X-Ethanol / (C2H5OH) / Ethyl Alcohol
- Y- Ethene / (C2H4)
- Z- Hydrogen/ (H2)
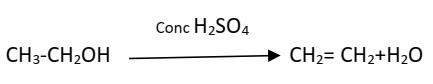
-
Role of sulphuric acid - dehydrating agent
- Exam Year: 2018
Related Questions
- Draw two different possible structures of a saturated hydrocarbon having four carbon atoms in its molecule
- Draw the electron-dot structure for ethyne. A mixture of ethyne and oxygen is burnt for welding
- Draw the structure of the following compounds
- Write the number of single and double covalent bonds present in a molecule of benzene
- Which compounds are called alkynes
- Differentiate between saturated and unsaturated carbon compounds