Decomposition reactions require energy either in the form of heat or light or electricity for breaking down the reactants
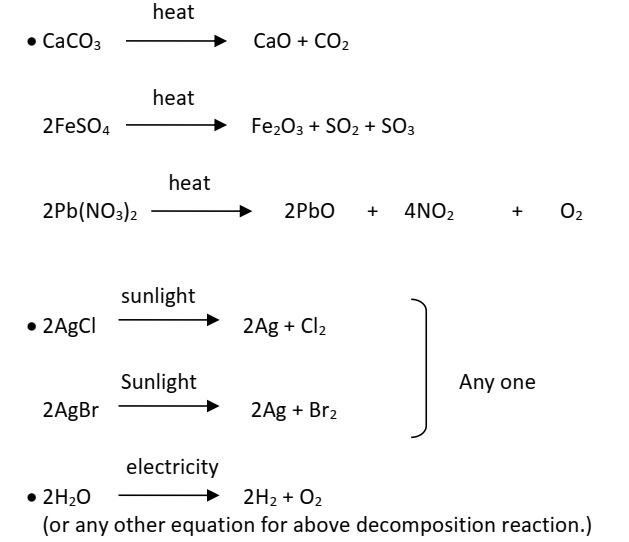
Chemical Reactions (10)Decomposition reactions require energy either in the form of heat or light or electricity for breaking down the reactants. Write one equation each for decomposition reactions where energy is supplied in the form of heat, light and electricity.
Answer
- Exam Year: 2018