On heating blue coloured powder of copper (II) nitrate in a boiling tube
Metals and Non-metals (10)On heating blue coloured powder of copper (II) nitrate in a boiling tube, black copper oxide, O2 and a brown gas X is formed.
- Identify the type of reaction and the gas X.
- Write balanced chemical equation of the reaction.
- Write the pH range of aqueous solution of the gas X.
Answer
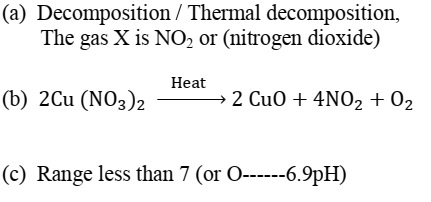
- Exam Year: 2019