The electrons in the atoms of four elements A, B, C and D are distributed in three shells
The electrons in the atoms of four elements A, B, C and D are distributed in three shells having 1, 3, 5 and 7 electrons respectively in their outermost shells. Write the group numbers in which these elements are placed in the Modern Periodic Table. Write the electronic configuration of the atoms of B and D and the molecular formula of the compound formed when B and D combine.
Answer
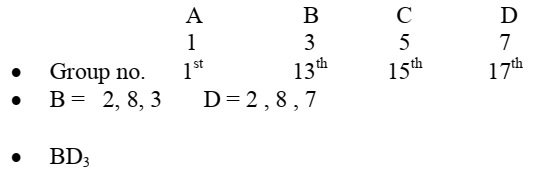
- Exam Year: 2019
Related Questions
- A student traces the path of a ray of light through a glass prism
- An aeroplane is flying at a height of 300 m above the ground
- Sumit is 3 times as old as his son
- Write all the values of p for which the quadratic equation $x^2 + px + 16 = 0$
- Which qualities of animals has the poet lost and now wants to regain? Answer with reference to the poem, Animals.
- Prove that: $\frac{\sin\theta} {\cot\theta +\csc\theta}$ = 2 + $\frac{\sin\theta}{\cot\theta - \csc\theta}$