How is copper extracted from its sulphide ore
Metals and Non-metals (10)How is copper extracted from its sulphide ore ? Explain the various steps supported by chemical equations. Draw labelled diagram for the electrolytic refining of copper.
Answer
Sulphide ore of copper is heated in air
2Cu2S+3O2 → 2Cu2O +2SO2
2Cu2O+Cu2S → 6Cu + SO2
Labelled diagram of electrolytic refining of copper
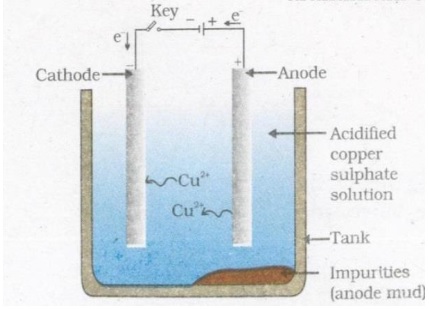
- Exam Year: 2018